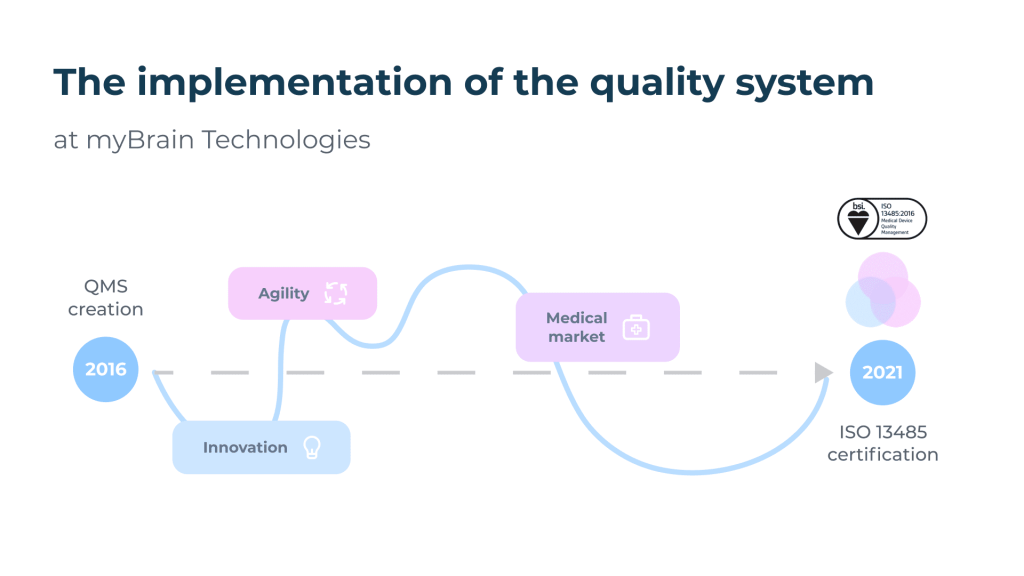
The path to ISO 13485.
By Sophie Colliot, Quality Manager at myBrain Technologies.

Implementing a quality approach within a start-up poses particular difficulties linked to the context of innovation, agility and rapid growth. However, such an approach is essential to guarantee the sustainability of its activities and to enable it to become a leader in its market.
Report.
On June 2, 2021, myBrain Technologies obtains the ISO 13485 certificate (Medical devices – Quality management systems – Requirements for regulatory purposes). The entire team is very proud of this. The publication of the ISO 13485 certificate is synonymous with easier access to the market of safe and efficient medical devices, but it also means for us the concretization of 5 years of beautiful, rich and intense experiences from an organizational and human perspective.

Launch.
The quality process was initiated in 2016 at myBrain Technologies, only two years after its creation, and following the completion of my second university degree « Managing Quality » in Specialized Master (ENSAM, Paris). As quality does not have a very good reputation in the startup world, it was necessary to transmit its strong points but also to adapt, innovate and advance step by step with the collaborators by training them, some of them having never had any experience in the medical field. The management team has always supported the quality process: it has appointed the pilots in a management review and set ambitious quality objectives. My role consisted first of all in establishing a relationship of trust with our service providers, our notified body BSI, as well as an ecosystem of internal and external skills to achieve our quality objectives.
Impact.
The quality process has had several positive impacts during its implementation. On the one hand, the « open » quality management system (QMS)[1] has facilitated the market access of melomind, a connected audio EEG device now marketed to learn how to manage one’s state of relaxation via the neurofeedback principle. On the other hand, it served as a springboard to develop our medical activity with the deployment of ISO 13485.
Methodology.
Today, we manage a totally dematerialized ISO 13485 QMS thanks to digital tools, which is a great advantage in the context of the health crisis: the first certification audits had to be conducted remotely. This specificity continues to be an advantage, since we can manage and improve our QMS remotely by maintaining a flexible organization in telecommuting.
Perspectives.
Our ISO 13485 process is conducted in parallel with the CE marking process according to the medical device regulation. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices (MDR) came into force on 25 May 2017 and came into effect on 26 May 2021 following the amending regulation (EU) 2020/561, i.e. 1 year later than initially planned. Our medical devices will be CE marked under the new MDR. BSI is one of the first organizations to be notified under the regulation. The marking of our medical devices is in progress with the collaboration of the whole team for the constitution of the technical file.
References.
[1] Duffaure S., Maranzana N., Attal Y. & Duchamp R. (2017). Démarche qualité et start-up. Déploiement au sein d’une start-up dans le domaine de la santé et du bien-être. Conférence : 12ème Congrès International de Génie Industriel (CIGI’17), Compiègne, France, Mai 2017.
Related Articles

A milestone for NeuroEthics
Neurotechnologies are no longer confined to research laboratories. They are fully integrated in our societies, whether in the medical field or beyond.
The benefits of collecting EEG neuroimaging insights in clinical trials.
In clinical trials, brain monitoring EEG is facilitating the measurement of complex clinical endpoints. EEG as a biomarker provides safe, objective an

Newsletter | December 2022.
During this year of 2022, we have dedicated ourselves to make our solution more complete and ergonomic through many updates designed to optimize th

Newsletter | November 2022.
The end of 2022 marks a major step forward in the neurotechnology sector in France: the presentation of a Charter for a responsible development of ne

A milestone for NeuroEthics.
The capacity of neurotechnologies to probe and interfere with brain activity challenges notions of identity, integrity and autonomy of human being. Re

Newsletter | October 2022.
To travel miles in full peace of mind, in a car that understands and anticipates every need of the driver on the road, is becoming a reality! With it

Newsletter | September 2022.
myBrain Technologies is committed to provide the most accurate offer possible to support its customers in the development of their products. With the

Newsletter | Summer 2022.
Statistics are here! Discover now our new statistic tools in Analytics to easily interpret your protocol results. Easily navigate, clearly see and qu

Newsletter | June 2022.
As a company that has developed a solution based on Artificial Intelligence (AI) and neuroscience ans as manufacturer of Medical Devices (MD), myBrain
![[DOWNLOAD] Artificial Intelligence Act: What future for AI in Europe?](https://emmanuel-guerin.fr/dev-mybraintech/wp-content/uploads/2022/06/white-paper-artificial-intellifence-act.png)
[DOWNLOAD] Artificial Intelligence Act: What future for AI in Europe?
With the increasing evolution of computing, robotics, and new technologies, AI is now a major part of our daily life. On April 21, 2021, the European
myBrain Technologies’s eye on Medical Device Regulation.
In order to improve health safety and harmonize rules within the EU, the Medical Device Regulation has been profoundly revised, resulting in new regul

Newsletter | May 2022.
Our brain still holds many secrets, both in its functionning and in its normal development. Thanks to the latest neurosciences techniques and tools, i

NeuroAeronautics: brain wave monitoring in the ‘Cockpit of the Future’.
A cockpit is a highly sensitive area, where attention and mental workload have to be optimized. Latest neurotechnologies, including EEG, can track bra
Newsletter | April 2022.
This month, the spotlight is on user experience that our Product Team keep on improving. We aim to focus on our customer's feedback to optimize our so

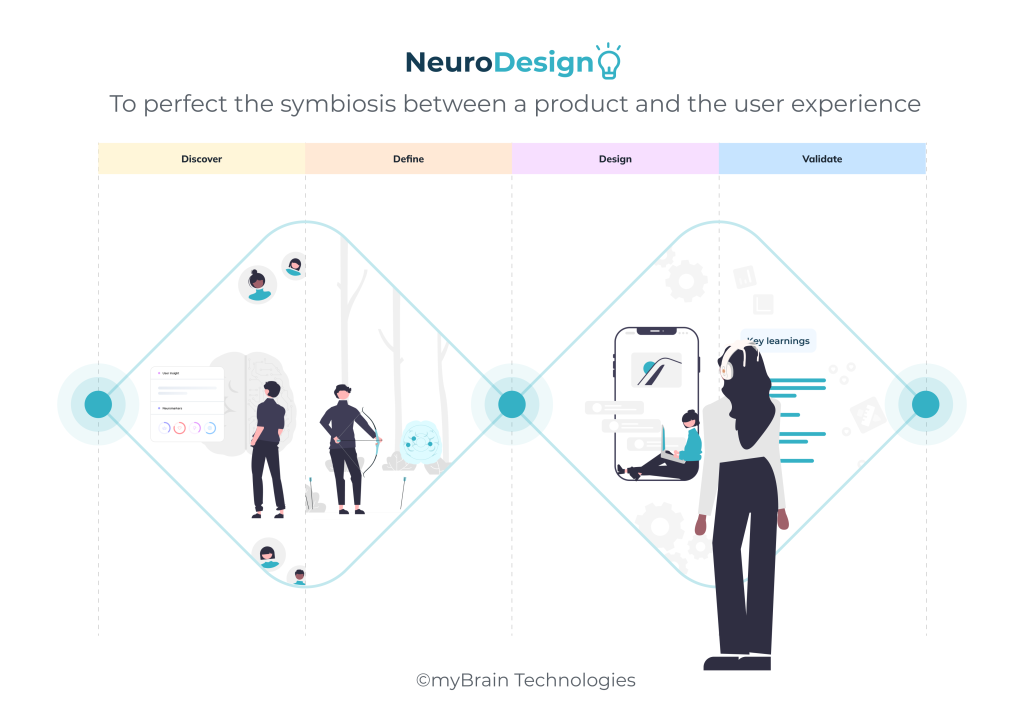
NeuroDesign: to perfect the symbiosis between a product and the user experience.
One of the key aspects of product strategy is to generate, identify and leverage insights that will provide the foundation to reach business objective

Newsletter | March 2022.
Hi there! The cosmetic industry has been interested for a while in exploring the links between the brain and the nervous system. The recent advances i

NeuroCosmetics in skincare.
Cutting edge “neuro-IA” technologies can detect the physiological impact of a cosmetic product using real-time consumers’ emotional data collect
Newsletter | February 2022.
First of all, we're deeply happy to have seen so many of you reading us last month and we thank you very much! Today, we are proud to announce that o

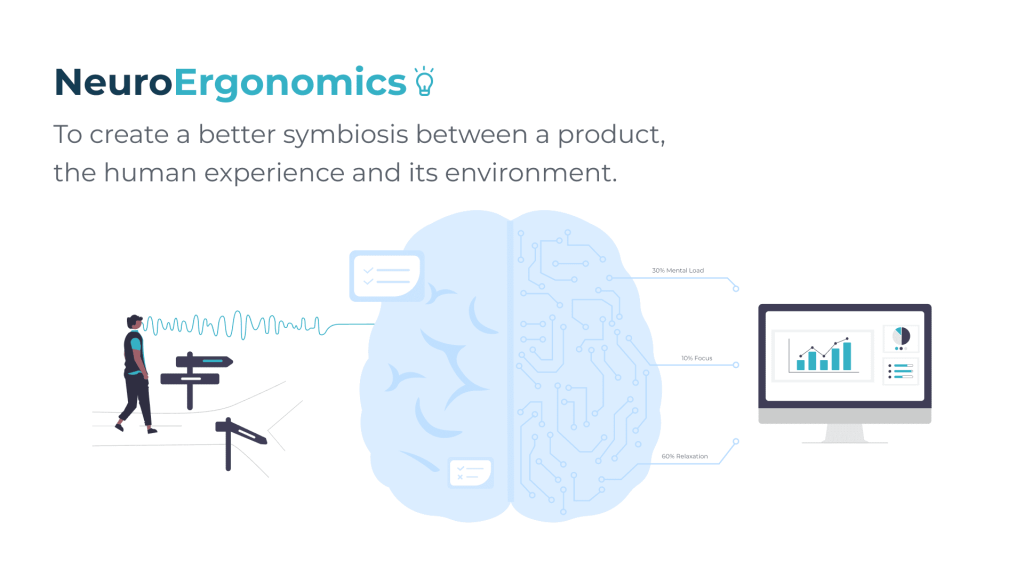
Neuroergonomics: towards a new paradigm of product design.
Neuroergonomics provides a multidisciplinary approach that merges elements of neuroscience, human factors, and ergonomics to study brain response to s

Newsletter | January 2022.
2021 has been full of exciting happenings. Since the very beginning of our adventure, we are dedicated to improve the life of others with neuroscienc

Press Release: Decoding the brain to understand human cognition and emotions.
myBrain Tech has developed its Neuromarkers platform to record, analyze and interpret the brain's electrical signals in real time. With this solution,

[SAVE THE DATE] – AI, conquering emotions in Health!
On Tuesday, November 16, 2021, at 11am, come and listen to Giuseppe Spinelli at the microphone of Pascal Becache on the occasion of the 4th Webinar #P
Computational Psychiatry: how neurotechnology is changing the world of mental health ?
Neuroscience and artificial intelligence are about to revolutionize medicine, especially psychiatry. Neurotechnologies offer a range of new tools base

The path to ISO 13485.
Implementing a quality approach within a start-up poses particular difficulties linked to the context of innovation, agility and rapid growth. However
Get in touch
We are glad you are interested in reaching out to us. Whether you have a question about our products or want to give us feedback, we are here to help.
