
Newsletter | June 2022.

.
As a company that has developed a solution based on Artificial Intelligence (AI) and neuroscience ans as manufacturer of Medical Devices (MD), myBrain Technologies has to stay up to date with regulations and standards in these fields.
For this, we can rely on our quality team! 🙏🏻
This month, we are proud to present the white paper of our Quality Director on the future of AI regulation in Europe 📗
We also talk about the difficulties and solutions related to the application of the Medical Device Regulation (MDR) 📃
Summary |
What future for AI in Europe? 📗 |
myBrain Tech’s standards 🏅 |
Eye on Medical Device Regulation 🎙 |
What future for AI in Europe?
#DownloadWhitePaper 📗

With the increasing evolution of computing, robotics, and new technologies, AI is now a major part of our daily life.
On April 21, 2021, the European Commission published a proposed regulation on AI, the Artificial Intelligence Act, which aims to establish safe and harmonized rules for this innovative field.
In her White Paper, Sophie Colliot, Quality Director at myBrain Technologies, reviews the main contributions, impacts and normative perspectives of the proposed regulation. She also compares the implementation of other regulations closely related to AI and specifies the various opinions of the CNIL and its counterparts on the subject, thus highlighting the possible fields of evolution.
Download the White Paper here >>
myBrain Tech’s standards for MDR
#Certification 🏅
At myBrain Technologies, we strive to ensure that our Quality Management System guarantees customer satisfaction through consistent and standardized processes together with centralized and organized quality control policies.

Focus on Medical Device Regulation
#Interview 🎙

Nowadays, if there is one sector in which AI systems are crating a major upheaval, it is the healthcare and Medical Devices sector.
With the aim of reinforcing health safety and harmonizing the application of the rules within EU, an in-depth revision of the regulations led to a new specific Medical Device Regulation (MDR 2017/745) – in application since May 26, 2021.
Bringing MDs to market, while complying with new regulatory requirements, poses challenges that legitimately worry manufacturers. However, there are strategies that can help them acquire the necessary certification to bring their products to market.
To understand these difficulties and find solutions, we interviewed Sophie Colliot, Quality Director at myBrain Technologies.
Discover the interview here >>

© 2022 myBrain Technologies, All rights reserved.
50 Avenue Claude Vellefaux
75010 Paris
Related Articles

A milestone for NeuroEthics
Neurotechnologies are no longer confined to research laboratories. They are fully integrated in our societies, whether in the medical field or beyond.
The benefits of collecting EEG neuroimaging insights in clinical trials.
In clinical trials, brain monitoring EEG is facilitating the measurement of complex clinical endpoints. EEG as a biomarker provides safe, objective an

Newsletter | December 2022.
During this year of 2022, we have dedicated ourselves to make our solution more complete and ergonomic through many updates designed to optimize th

Newsletter | November 2022.
The end of 2022 marks a major step forward in the neurotechnology sector in France: the presentation of a Charter for a responsible development of ne

A milestone for NeuroEthics.
The capacity of neurotechnologies to probe and interfere with brain activity challenges notions of identity, integrity and autonomy of human being. Re

Newsletter | October 2022.
To travel miles in full peace of mind, in a car that understands and anticipates every need of the driver on the road, is becoming a reality! With it

Newsletter | September 2022.
myBrain Technologies is committed to provide the most accurate offer possible to support its customers in the development of their products. With the

Newsletter | Summer 2022.
Statistics are here! Discover now our new statistic tools in Analytics to easily interpret your protocol results. Easily navigate, clearly see and qu

Newsletter | June 2022.
As a company that has developed a solution based on Artificial Intelligence (AI) and neuroscience ans as manufacturer of Medical Devices (MD), myBrain
![[DOWNLOAD] Artificial Intelligence Act: What future for AI in Europe?](https://emmanuel-guerin.fr/dev-mybraintech/wp-content/uploads/2022/06/white-paper-artificial-intellifence-act.png)
[DOWNLOAD] Artificial Intelligence Act: What future for AI in Europe?
With the increasing evolution of computing, robotics, and new technologies, AI is now a major part of our daily life. On April 21, 2021, the European
myBrain Technologies’s eye on Medical Device Regulation.
In order to improve health safety and harmonize rules within the EU, the Medical Device Regulation has been profoundly revised, resulting in new regul

Newsletter | May 2022.
Our brain still holds many secrets, both in its functionning and in its normal development. Thanks to the latest neurosciences techniques and tools, i

NeuroAeronautics: brain wave monitoring in the ‘Cockpit of the Future’.
A cockpit is a highly sensitive area, where attention and mental workload have to be optimized. Latest neurotechnologies, including EEG, can track bra
Newsletter | April 2022.
This month, the spotlight is on user experience that our Product Team keep on improving. We aim to focus on our customer's feedback to optimize our so

NeuroDesign: to perfect the symbiosis between a product and the user experience.
One of the key aspects of product strategy is to generate, identify and leverage insights that will provide the foundation to reach business objective

Newsletter | March 2022.
Hi there! The cosmetic industry has been interested for a while in exploring the links between the brain and the nervous system. The recent advances i

NeuroCosmetics in skincare.
Cutting edge “neuro-IA” technologies can detect the physiological impact of a cosmetic product using real-time consumers’ emotional data collect
Newsletter | February 2022.
First of all, we're deeply happy to have seen so many of you reading us last month and we thank you very much! Today, we are proud to announce that o

Neuroergonomics: towards a new paradigm of product design.
Neuroergonomics provides a multidisciplinary approach that merges elements of neuroscience, human factors, and ergonomics to study brain response to s

Newsletter | January 2022.
2021 has been full of exciting happenings. Since the very beginning of our adventure, we are dedicated to improve the life of others with neuroscienc

Press Release: Decoding the brain to understand human cognition and emotions.
myBrain Tech has developed its Neuromarkers platform to record, analyze and interpret the brain's electrical signals in real time. With this solution,

[SAVE THE DATE] – AI, conquering emotions in Health!
On Tuesday, November 16, 2021, at 11am, come and listen to Giuseppe Spinelli at the microphone of Pascal Becache on the occasion of the 4th Webinar #P
Computational Psychiatry: how neurotechnology is changing the world of mental health ?
Neuroscience and artificial intelligence are about to revolutionize medicine, especially psychiatry. Neurotechnologies offer a range of new tools base
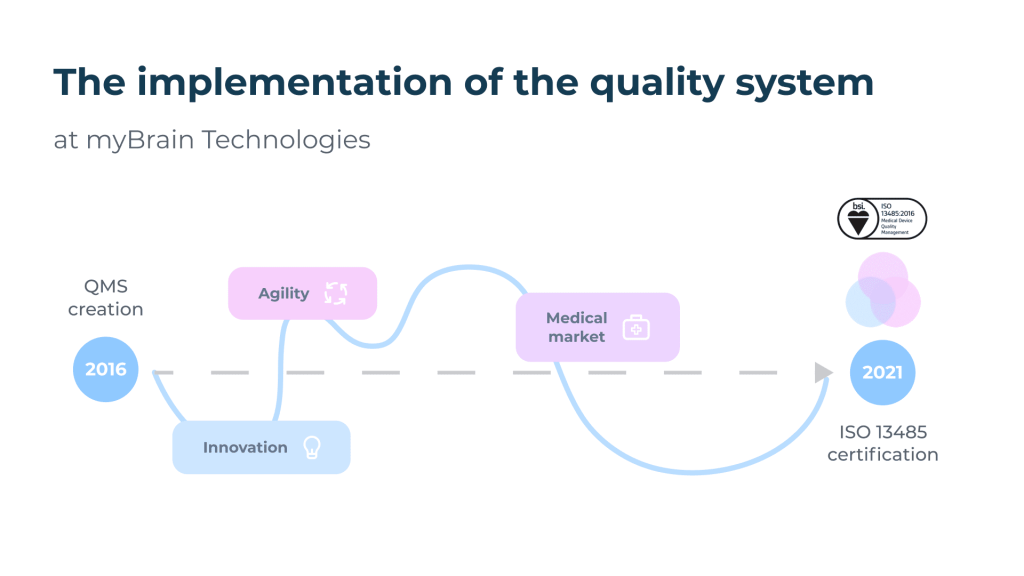
The path to ISO 13485.
Implementing a quality approach within a start-up poses particular difficulties linked to the context of innovation, agility and rapid growth. However
Get in touch
We are glad you are interested in reaching out to us. Whether you have a question about our products or want to give us feedback, we are here to help.

Laisser un commentaire